So I started looking into decaffeination methods for tea and coffee again/more. And it’s disturbing.
Here’s what I’ve found:
decaf tea uses 1 of 3 methods:
solvent, CO2, hot water.
if it says “organic” it’s c02. hot water isn’t certified organic. idk why.
legally, decaf tea or coffee must remove at least 97% of caf. otherwise you can’t call it decaf.
the hot water method only removes like 85% of caf, so I think it’d have to be sold as “reduced caf” (and non-organic), so it just isn’t being used even though I think it’s safe, cheap and good.
the CO2 method is also good from what i’ve seen so far. lipton and twinnings use the solvent method for their decaf tea.
decaf regulations are not enforced well and aren’t followed consistently. this is apparently just a general complaint about the industry.
i found that lipton doesn’t care about following the law:
Buy Lipton Decaf Herbal Black Tea Bags | Lipton US
Hi Myreall, thank you for contacting Lipton. The term decaffeinated refers to the removal of caffeine from the tea leaf; therefore, this process lessens the amount of caffeine left in the tea. Decaffeinated products contain caffeine but a much smaller amount than their regular caffeine counterparts. The Lipton Decaf Black Tea contains less than 5 mg of caffeine per 8 oz cup serving. Feel free to reach out to our team at 1-888-547-8668 if you have any additional questions. Thanks!
Since a cup of tea generally has under 100mg of caf, they need to get it down to 3mg or probably less to call it “decaf”. I did see black tea as up to 120mg of caf on some chart, which still isn’t the 167+mg necessary for under 5mg to legally be “decaf”.
Lipton also answered what method they use for decaf (my bold):
Hello David, We use a decaffeination process that retains our signature flavor. We use the ethyl acetate method to decaffeinate our tea. The process involves ethyl acetate to remove the caffeine out of the tea leaf; afterward, the ethyl acetate is removed from the leaf, and the leaves are dried. There are no safety concerns with the use of ethyl acetate. Ethyl acetate is an organic compound found in tea leaves, fruits, and coffee, and is one of the most popular ways to decaffeinate tea and coffee. I hope this was helpful!
Similarly Twinnings says:
Twinings teas are decaffeinated using the ethyl acetate method, which is one of the most popular ways to decaffeinate coffee and tea. After the tea leaves are moistened with water or steam, the process selectively absorbs caffeine and removes it from the tea. Afterward, ethyl acetate is removed, and the leaves dried. Ethyl acetate is an organic compound that is found in tea, coffee, and fruit, and provides for a safe and effective decaffeination method.
Is this safe? Lipton says it’s we should accept it as safe because it’s “one of the most popular ways to decaffeinate tea and coffee” and has “no safety concerns” (that statement is egregious fraud, isn’t it? it is obviously factually false that there are “no safety concerns”. Twinnings instead said it is “safe” which isn’t such an absolute statement, and also is about the product itself instead of the allegedly non-existence of concerns, so it doesn’t seem like fraud unless they actually knew it was unsafe or should have known.)
Lipton also said:
Ethyl acetate is highly flammable, as well as toxic when ingestion or inhaled, and this chemical can be seriously damaging to internal organs in the case of repeated or prolonged exposure. Ethyl acetate can also cause irritation when it comes into contact with the eyes or skin. My question is how much ethyl acetate is left on the tea leaves, as it is impossible to remove 100% of this chemical?
The amount of ethyl acetate left in the final product is nominal and harmless. Ethyl acetate is a naturally occurring compound found in fruits such as bananas. Bananas are non-toxic and non-flammable when consumed under ordinary circumstances by reasonable people (and apes). You should have as much concern over the ethyl acetate in your decaffeinated tea as you would about your bananas exploding. Hope this calms your concerns!
This seems misleading since I believe Wikipedia when it says:
[Ethyl acetate is] poisonous when inhaled or ingested.
We’re talking about a solvent used in glues and nail polish remover.
Since that sounds bad, I’m wondering if I’m better off drinking regular green tea than decaf tea made with ethyl acetate. You know what I’d prefer to that? The hot water method. That sounds cheap, safe, and good enough. I’d much rather get rid of 85% of the caf than none. Our civilization is really dumb sometimes. So far I don’t know how/where to buy any CO2 method decaf tea either.
Apparently they like to write “naturally decaffeinated” because ethyl acetate is extracted from a fruit. On a related note, I’m skeptical of Stevia, Monkfruit and some other plant extracts. Just because a whole plant is OK doesn’t mean some concentrated extract is.
Food labelling requires stating added caffeine (like for energy drinks that add it on purpose) but doesn’t require information about caffeine that’s there normally (like in tea or coffee).
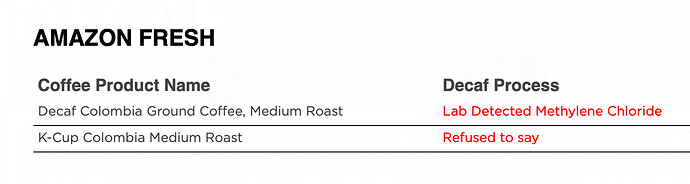
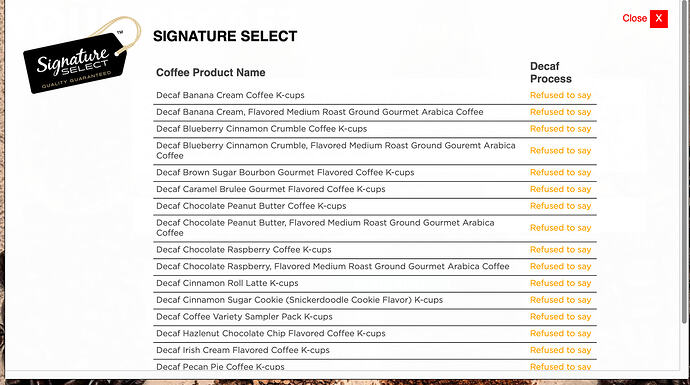
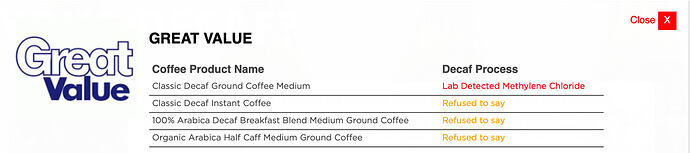
So far I’ve been unable to find out what method Safeway’s Signature Select brand uses for it’s decaf green tea. Refusing to give information about the food you’re selling is apparently a thing. Lab testing is often required to find out what big companies are selling.
https://checkyourdecaf.org
Starbucks says:
https://athome.starbucks.com/learn/what-is-decaffeinated-coffee
How Is Decaf Coffee Made?
The decaffeination process begins with green coffee beans after they are harvested. The green coffee beans naturally have a hard interior and exterior when first picked from the trees. Once picked, there are three primary ways to create decaffeinated coffee: the Direct Contact Method, the SWISS WATER® Process and the Natural Decaffeination Process. Each of these methods provides a safe and effective way to remove 97% or more of the caffeine from the coffee beans, per the high standard put in place by The U.S. Food and Drug Administration.?
The Direct Contact Method is the most common way to decaffeinate coffee effectively. During this process, green coffee beans are steamed to open their “pores.” Once the beans are sufficiently softened, a solvent is added to the mass of wet beans. Caffeine molecules then bond with the solvent, leaving the bulk of the other flavor compounds intact. Once the caffeine has been removed, the beans are washed, steamed and roasted at over 400°F to evaporate all liquids used in this process.
The SWISS WATER® Process removes caffeine from green coffee beans by soaking them in warm water to create “flavor-charged water.” That water is then run through an activated charcoal filter that captures the caffeine molecules. No solvents are applied directly to the coffee bean, but the carbon filter essentially filters out the caffeine. Then the coffee beans are soaked in the flavor-charged water to reintroduce the flavors to the coffee.
The Natural Decaffeination Process starts with water-soaked green coffee beans sealed in a stainless-steel tank. Liquid carbon dioxide is forced into the tank at a very high pressure. This process draws out and dissolves the caffeine, leaving larger flavor molecules behind.
Regardless of the process chosen, one step that’s always shared is that the beans are prepared by soaking them in warm water to soften them prior to roasting. This increase of moisture makes the caffeine more easily removable while still preserving the flavor you love in the bean. Once the caffeine is extracted or dissolved, our skilled roasters bring out the aroma, acidity, body and flavor within the decaffeinated beans. Voila! You now have a delicious decaffeinated cup of your favorite Starbucks® coffee.
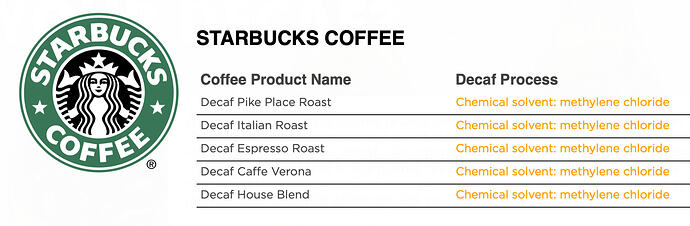
This is very misleading. They only use one of these methods and they don’t say that nor say which one it is.
Starbucks uses methylene chloride for their “direct contact” solvent. They at least admit this (elsewhere) rather than refusing to say. I saw it in an article and
Is methylene chloride bad?
https://www.chemicalsafetyfacts.org/methylene-chloride/
Methylene chloride is most prominently used industrially — in the production of paint strippers, pharmaceuticals and process solvents.
- Methylene chloride also is used in processing spices, creating hops extract for beer and other flavorings for the food and beverage industries.
So all sorts of spices I buy could have it, too…
OSHA’s Methylene Chloride Standard sets a permissible exposure limit of 25 parts of methylene chloride per million parts of air over an eight-hour period.
I was trying to figure out if it’s poisonous. That sounds probably pretty poisonous… 25 parts per million is a low amount.
Methylene chloride poisoning incidents during paint stripping operations and bath tub refinishing have demonstrated that inhalation exposure at extremely high levels can be fatal to humans.
OK so it can kill you.
Effective November 24, 2019, it will be illegal to manufacture (including import), process, distribute or sell methylene chloride in paint removers for consumer use. EPA found consumer uses of the chemical in paint strippers to pose unreasonable risks to human health.
So you can’t put it in paint removers for consumers anymore, but it’s still OK to use for our decaf Starbucks coffee!?
CFR - Code of Federal Regulations Title 21
Methylene chloride may be present in food under the following conditions:
I’m not sure if it’s banned from all other foods that aren’t mentioned here or if it’s allowed in them without limits. They should write this stuff more clearly.
In spice oleoresins as a residue from the extraction of spice, at a level not to exceed 30 parts per million
That’s more than the 25ppm limit in the air from earlier.
In coffee as a residue from its use as a solvent in the extraction of caffeine from green coffee beans, at a level not to exceed 10 parts per million (0.001 percent) in decaffeinated roasted coffee and in decaffeinated soluble coffee extract (instant coffee).
That’s 40% of the air limit. And what is being done to measure and make sure Starbucks doesn’t have too much? It’s hard to be comfortable with this without knowing who is measuring what, on what schedule, with what methods, etc., to make sure this law is actually followed. Starbucks ought to be measuring this stuff themselves and sharing information about their safety procedures (if they have good ones…). Instead I’ve repeatedly read companies saying stuff like “we have very high safety standards since we follow the laws [and nothing more]” with no details to convince me they actually do follow the laws or that they put any thought into whether the laws are actually good/high/safe/appropriate standards.
I found a reason this is allegedly safe:
What Is Decaf Coffee?
When the coffee is roasted, both the solvent and the caffeine are burnt away. (The boiling point of methylene chloride is about 100 F and Starbucks roasts its coffee at 375 to 475 F.)
This page also says:
a [Starbucks] Pike’s Place blend contains 310 [milligrams of caffeine]
and
a grande decaf Pike’s Place brewed coffee contains 25 milligrams of caffeine.
25/310 is 8% caffeine remaining, which would be illegal if true. A drink with 310mg of caf needs to get under 9.3mg of caf (3%) to be “decaf”. The article authors don’t seem aware that they’re accusing Starbucks of breaking the law. Kind of like Lipton seems unaware that they publicly post claims that they break the law.
The article authors also seem unaware that it’s a “Pike Place Ground Roast” from Starbucks. That’s “Pike” not “Pike’s”. https://athome.starbucks.com/products/pike-place-roast-ground I just wanted to verify there is a drink from Starbucks by that name, to do some basic fact checking, since I don’t remember hearing of the drink before. I wanted to make sure I wasn’t confused in case e.g. Pike’s Place was a different coffee shop that was also being discussed in the article. (Actually Pike Place is a market in Seattle and the first Starbucks was opened there.) I wasn’t actually trying to catch them out but I did by accident which is kinda sad.
Anyway, tons of stuff sucks and is a lot of work to look up effectively (I still don’t have good answers).